Cardiac
programs
Advancing Gene Therapy in Cardiovascular genetic diseases
Despite recent medical advances in the precision cardiovascular field, cardiovascular disease remains the most significant cause of morbidity and mortality in the western world and is rapidly becoming a primary cause of death worldwide. Therefore, there is an urgent need for new treatments to address these conditions for patients.
We are developing a number of disease-modifying gene therapy candidates to treat larger-rare cardiovascular diseases that have significant unmet need and no approved treatments that address the underlying genetic cause of the disease.
CARDIOVASCULAR PROGRAMS
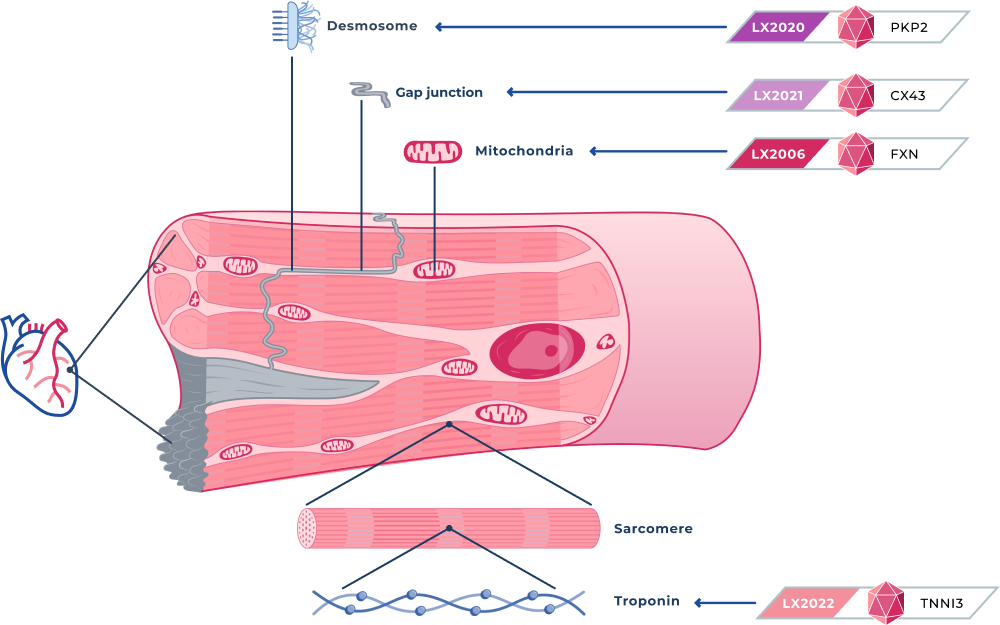
LX2006
Cardiac Friedreich’s Ataxia
LX2006 is a gene therapy candidate designed to deliver a functional frataxin, or FXN, gene for the treatment of FA cardiomyopathy. FA cardiomyopathy is the most common cause of mortality in patients with FA and affects approximately 5,000 patients in the United States.
LX2006 is designed to promote the expression of the protein frataxin to restore normal mitochondrial function and energy production in myocardial cells.. LX2006 is currently being evaluated in an open-label, ascending dose Phase 1/2 clinical trial in patients with FA cardiomyopathy (SUNRISE-FA).
The FDA has granted Rare Pediatric Disease designation and Orphan Drug designation to LX2006 for the treatment of FA.
LX2020
Arrhythmogenic cardiomyopathy (PKP2)
LX2020 is a gene therapy candidate designed to deliver a fully functional PKP2 gene to cardiac muscle for the treatment of PKP2-ACM. PKP2 mutations are associated with approximately 75% of all genetic cases of ACM, and we estimate they affect approximately 60,000 patients in the United States. PKP2 mutations can cause replacement of heart muscle with fibrotic tissue and fatty deposits, and severe abnormal heart rhythms, or arrhythmias, that cause cardiac dysfunction and can result in sudden cardiac death.
LX2020 is designed to increase desmosomal PKP2 protein levels, reassemble desmosomes and restore myocardial cell function.
LX2021
DSP (Desmoplakin) cardiomyopathy (CX43)
LX2021 is a gene therapy candidate we are developing to deliver a functional connexin 43, or Cx43, protein for a group of inherited cardiac muscle disorders associated with a high risk of sudden death, including ACM and certain forms of dilated cardiomyopathy. We believe restoring the Cx43 protein can potentially treat multiple genetic causes of ACM because the cardiac loss of Cx43 is a molecular deficit generally observed in all ACM patient populations.
Our LX2021 program is initially targeting Desmoplakin, or DSP, cardiomyopathy, a distinct form of ACM as well as a certain form of dilated cardiomyopathy, impacting up to approximately 35,000 patients in the United States.
LX2022
Hypertrophic cardiomyopathy (TNNI3)
LX2022 is a gene therapy candidate we are developing to deliver a functional TNNI3 gene to myocardial cells to treat a distinct form of hypertrophic cardiomyopathy, or HCM, due to mutations in the TNNI3 gene. Mutations in the TNNI3 gene often result in left ventricular hypertrophy and restrictive cardiomyopathy, leading to arrhythmias and heart failure.
With an estimated prevalence of 1 in 500 people in the United States, HCM is one of the most common forms of genetic cardiomyopathy and is caused by mutations that affect the cardiac sarcomere in approximately 75% of cases